What is Mono Ethylene Glycol?
Mono ethylene glycol (also known as MEG, EG, 1,2-ethanediol or 1,2-Dihydroxyethane) is an organic compound with the formula C2H6O2. It is a slightly viscous liquid with a clear, colourless appearance and a sweet taste that emits virtually no odour. It’s miscible with water, alcohols, and many other organic compounds and is primarily used in the industry for manufacturing polyester fibres and as a component in the production of antifreeze, coolants, aircraft anti-icers and de-icers.
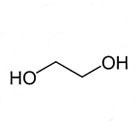
Technical Properties
Technical properties of Mono Ethylene Glycol:
Molecular Formula: C2H6O2 / (CH2OH)2 / HOCH2CH2OH
Synonyms: monoethyleneglycol, mono ethyl glycol, meg glycol, ethylene glycol, 1,2-ethanediol, Ethane-1,2-diol, EG, industrial glycol, 1,2-Dihydroxyethane, glycol alcohol.
Cas Number: 107-21-1
Molecular Mass: 62.07 g/mol
Exact Mass: 62.036779 g/mol
Flashpoint: 232 °F/ 111.11 °C
Autoignition temperature: 770 °F / 410 °C
Boiling Point: 387.7 °F / 197.6 °C at 760 mm Hg
Melting Point: 9 ° F / -12.8 °C
Vapour Pressure: 0.06 mm Hg at 68 °F / 20 °C
Density: 1.115 at 68 °F
Log P: -1.69
How is Mono Ethylene Glycol Produced?
The intermediate ethylene oxide is used to convert ethylene (ethene) into ethylene glycol. Ethylene oxide is obtained through oxidation and is then reacted with water to give mono ethylene glycol with di and tri ethylene glycols as co-products:
C2H4O + H2O → HOCH2CH2OH
Acids and bases can catalyse this reaction, or it can occur at neutral pH under elevated temperatures. Yields of up to 90% can be achieved with acidic or neutral pH with a large excess of water.
Mono ethylene glycol is also manufactured via the hydrogenation of dimethyl oxalate in the presence of a copper catalyst or via the acetoxylation of ethylene.
Global demand for Monoethylene Ethylene Glycol (MEG) is strong with the market worth $25 billion and expected to grow 6% annually to 2024. This is especially due to the increased production of polyethene terephthalate (PET) and the demand for polyesters in the Asia Pacific. Demand is strongest in China where approximately 70% of the world’s MEG output is consumed.
How is Mono Ethylene Glycol Stored and Distributed?
Storage & Handling
Under the NFPA 704, mono ethylene glycol is rated as a 0 for instability, indicating that mono ethylene glycol is usually stable. Mono ethylene glycol’s vapours are heavier than air and will travel to surrounding areas. Due to its high flashpoint of 111.11°C, the chemical has a flammability rating of 1, indicating that it requires considerable preheating for ignition and combustion to occur. However, in storage, MEG should be kept away from heat, sparks, and open flames. If a fire was to occur, alcoholresistant foam or water spray should be used to fight fires with a focus on preventing the spillage from reaching water sources or sewers.
Mono Ethylene Glycol Health Hazards
The chemical has high toxicity when ingested with the major danger being a result of the sweet taste encouraging further consumption; this increases the danger posed to animals and children. Ingestion of sufficient amounts is fatal if left untreated with the ethylene glycol being oxidised in the body to glycolic acid and then the toxic chemical, oxalic acid. MEG ingestion impacts the central nervous system, heart and can cause acute kidney failure.
Eye exposure to mono ethylene glycol vapours can irritate, and therefore it is suggested goggles should be worn while handling the chemical. Exposure to ethylene glycol in liquid form has the potential to cause more serious eye damage. If contact is made with the eyes, immediately wash with plenty of water and seek medical attention.
Skin exposure with MEG can also irritate and so gloves should be worn. If the skin does become contaminated, all wet clothing should be removed, and the skin washed with water. Inhalation exposure to high levels of ethylene glycol can cause irritation, and potentially intolerable respiratory discomfort and coughs. If excessive inhalation occurs, the individual should be removed from the environment, breathe fresh air, and seek medical attention.
Mono Ethylene Glycol Uses
Mono ethylene glycol is more commonly used as a polymer precursor but also in antifreeze as well as in a wide variety of industries.
Polymer Precursor
There is strong global demand for mono ethylene glycol in the plastic industry as it is a vital ingredient in the production of polyester fibres, films, and resins, one of which is polyethene terephthalate (PET). PET is created by heating ethylene glycol with terephthalic acid in an esterification reaction. This chemical is then converted into plastic bottles, microwaveable containers and is even used in the textile industry. It is estimated that 70-80% of all the MEG consumed is used as a chemical intermediate in these polyester production processes.
Antifreeze
A primary industry use of mono ethylene glycol is in antifreeze applications where it is a component in the manufacture of antifreeze, coolants, aircraft anti-icers and deicers due to its ability to depress the freezing temperature of the water. While pure ethylene glycol freezes at -12.9 °C, when mixed with water this can greatly reduce to around -45 °C with 60% ethylene glycol and 40% water. Bitter flavourings are usually added to MEG used in antifreeze to reverse the sweet taste which may encourage children and animals to consume the chemical.
Other Industry Uses
Mono ethylene glycol is also used in the manufacture of alkyd resins which are used to form a film in paints, enamels, and varnishes. In the gas industry, ethylene glycol is used to remove water vapour from the gas before it is processed further. Here, it is also used as a desiccant in gas pipelines to stop the formation of clathrates.
For the electronic industry, MEG is used as a chemical intermediate in the production of capacitors. Or for the treatment and prevention of fungi and rot in wood, mono ethylene glycol can be used, especially useful for exhibitions and museums. In the medical field, ethylene glycol can be used in the production of vaccines, although it is not present in the final administered vaccine. It is a minor ingredient in a variety of cleaners, including screen cleaners for electronics where it is paired with isopropyl alcohol.
Solventis as your Mono Ethylene Glycol Supplier
Solventis is a leading bulk supplier and distributor of mono ethylene glycol or MEG in the UK, Europe and globally. As a company with offices in the UK and Belgium, we are proud to be able to offer a personal and efficient service, together with highly competitive prices.
As a result of our state-of-the-art facility in Antwerp, Belgium, we can guarantee the quality, efficiency, safety, and environmental awareness for our whole range of products that is second to none. Please contact us for more information on our mono ethylene glycol or any glycol from our range on our enquiry form or by phone on +44 (0)1483 203224.



