What is Methyl Glycol?
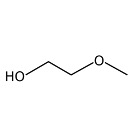
Methyl glycol (also known as 2-methoxyethanol, glycol monomethyl ether, ethylene glycol monomethylether, methyl cellosolve, methyl oxito, and MGL) is a clear, colourless liquid that has a mild characteristic, ether-like, odour. It is miscible with water and many organic solvents and has the formula C3H8O2
How is it produced?
Methyl glycol is produced via the reaction of ethylene oxide with methanol, in water, and in a high temperature and high pressure environment.
How is it stored and distributed?
Methyl glycol has a flashpoint of 37 °C (closed cup) and is thus highly flammable and should be stored in a cool, dry, well-ventilated area that is free from the possibility of ignition. For transport purposes it is categorised as hazard class 3, and packing group III. 2-methoxyethanol is classified as harmful and has a specific gravity of 0.965.
2 Methoxyethanol Uses
Methyl glycol has the ability to dissolve a variety of different types of chemical compounds. This ability sees it used as a solvent for cellulose acetate, and a solvent for resins (particularly in the electronics industry). 2-methoxyethanol is used in alcohol soluble dyes, and in quick-drying varnishes, enamels, nail polishes and wood stains.
It is also used as an additive in aeroplane deicing solutions and small amounts of methyl glycol are also used in perfume fixatives and in the manufacture of photographic film.



